Soil Organic Matter (SOM)
- Introduction
- Soil Organic Matter (SOM) is the organic fraction of the soil, consisting of living organisms, dead plant and animal materials, and their decomposed products.
Though SOM usually constitutes only 1–5% of soil by weight, it has an extraordinary influence on soil fertility, structure, microbial activity, and environmental sustainability. - It serves as a source and sink of plant nutrients, contributes to soil structure and water retention, and plays a vital role in the carbon and nitrogen cycles of ecosystems.
- In essence: “Soil organic matter is the life and soul of soil fertility.”
- Definition
- According to the Soil Science Society of America (SSSA): “Soil organic matter is the organic fraction of the soil that includes plant and animal residues at various stages of decomposition, cells and tissues of soil organisms, and substances synthesized by soil organisms.”
- Simplified Definition: SOM is the sum total of all organic components in the soil — living, dead, and decomposed — derived from plant, animal, and microbial sources.
- Composition of Soil Organic Matter
SOM is a complex mixture of organic materials at different stages of decomposition.
|
Component |
Nature |
Approximate % of Total SOM |
|
Living biomass |
Microbes (bacteria, fungi, actinomycetes), roots, fauna |
5–10% |
|
Fresh residues |
Recently added plant and animal materials |
10–20% |
|
Active (labile) fraction |
Partially decomposed materials; easily decomposable |
20–40% |
|
Stable (passive) fraction / Humus |
Fully decomposed, resistant to further decay |
40–60% |
- Chemical Composition of SOM
SOM is rich in elements essential for plant and microbial life.
|
Element |
Percentage (%) |
|
Carbon (C) |
50–58 |
|
Oxygen (O) |
30–40 |
|
Hydrogen (H) |
3–5 |
|
Nitrogen (N) |
3–5 |
|
Sulphur (S) |
<1 |
|
Phosphorus (P) |
<1 |
- Sources of Soil Organic Matter
- Plant residues — leaves, stems, roots, bark, litter, and exudates.
- Animal remains — dung, urine, carcasses, and other organic wastes.
- Soil organisms — microbes and soil fauna (bacteria, fungi, worms).
- Root exudates — sugars, amino acids, and organic acids secreted by roots.
- Organic amendments — compost, FYM, green manure, and biochar.
Root residues often contribute more to SOM than above-ground residues.
- Fractions of Soil Organic Matter
|
Fraction |
Description |
Decomposition Rate |
|
Active (Labile) SOM |
Easily decomposable; provides nutrients quickly |
Rapid |
|
Slow SOM |
Intermediate; decomposes over years |
Moderate |
|
Passive (Stable) SOM / Humus |
Resistant to decomposition; acts as long-term nutrient reservoir |
Very slow |
7. Processes of Organic Matter Transformation;
SOM is continuously formed, decomposed, and transformed through biological processes.
The major processes include:
- Decomposition; Breakdown of complex organic materials into simpler compounds through microbial and faunal activity.
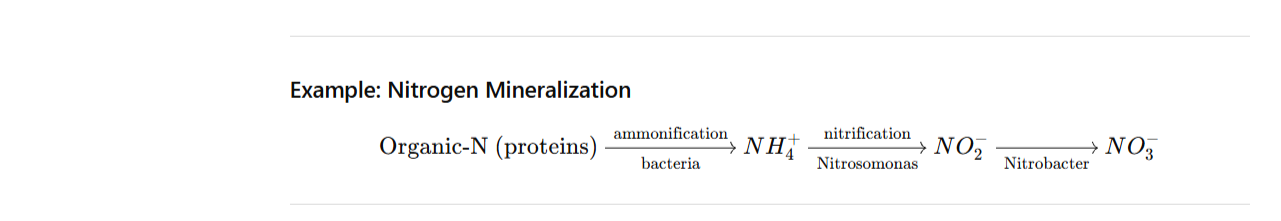
- Mineralization; Conversion of organic nutrients into inorganic, plant-available forms (e.g., N → NH₄⁺ → NO₃⁻).
- Humification; Transformation of decomposed organic matter into stable, complex organic substances (humus).
- Immobilization; Temporary tying up of inorganic nutrients (like N and P) into microbial biomass.
- Decomposition Process
Stages:
- Fragmentation — Physical breakdown of residues by fauna (earthworms, termites).
- Leaching — Soluble organic compounds (sugars, amino acids) move downward with water.
- Catabolism — Microbial oxidation of organic compounds, releasing CO₂ and energy.
- Humification — Formation of humic substances (humic and fulvic acids).
- Mineralization — Release of nutrients (N, P, S) into inorganic forms.
Microorganisms Involved
|
Group |
Function |
|
Bacteria |
Decompose proteins, sugars, starches |
|
Fungi |
Break down cellulose, hemicellulose, lignin |
|
Actinomycetes |
Decompose resistant materials |
|
Fauna |
Mix and aerate soil (earthworms, nematodes) |
Example: Nitrogen Mineralization
- C:N Ratio (Carbon to Nitrogen Ratio)
Definition: The ratio of carbon to nitrogen in organic materials.
Importance: Determines the rate of decomposition and availability of nitrogen.
|
Material |
C:N Ratio |
|
Green manure |
15–20:1 |
|
Farmyard manure |
20–25:1 |
|
Straw |
60–80:1 |
|
Humus |
10–12:1 |
Interpretation:
- Narrow ratio (<20:1): Rapid decomposition, nitrogen released (mineralization).
- Wide ratio (>30:1): Slow decomposition, nitrogen immobilized (temporary deficiency).
- Humification
Definition: The process of formation of stable, dark-colored, high-molecular-weight organic compounds (humus) through microbial decomposition and chemical synthesis.
Stages:
- Decomposition of residues.
- Formation of intermediate organic compounds (phenols, amino acids).
- Condensation/polymerization → humic substances.
- Stabilization → humus.
Humus Composition:
- Humic acid: Soluble in alkali, precipitates in acid.
- Fulvic acid: Soluble in both acid and alkali.
- Humin: Insoluble in both.
- Mineralization
Definition: Conversion of organic forms of nutrients into inorganic, plant-available forms.
|
Element |
Organic Form |
Inorganic Form Released |
|
N |
Proteins, amino acids |
NH₄⁺, NO₃⁻ |
|
P |
Nucleic acids, phospholipids |
H₂PO₄⁻, HPO₄²⁻ |
|
S |
Sulfhydryl groups |
SO₄²⁻ |
|
C |
Carbohydrates, fats |
CO₂ |
- Factors Affecting SOM Decomposition
|
Factor |
Effect |
|
Temperature |
Warm climates accelerate decomposition |
|
Moisture |
Optimal at 60–80% of field capacity |
|
Aeration |
Aerobic conditions favor faster breakdown |
|
Soil pH |
Neutral pH (~6.5–7.5) is optimal |
|
Residue composition |
Sugars, proteins decompose faster than lignin |
|
C:N ratio |
Narrow ratio → faster decomposition |
|
Soil texture |
Clay protects SOM from rapid decay |
|
Management practices |
Tillage and residue burning accelerate SOM loss |
13. Functions and Importance of Soil Organic Matter
- Physical Functions
|
Property |
Effect of SOM |
|
Soil structure |
Promotes aggregation and porosity |
|
Water retention |
Increases soil’s capacity to hold water |
|
Soil color |
Darkens soil, aiding heat absorption |
|
Erosion control |
Improves aggregate stability |
|
Bulk density |
Lowers soil density, enhancing aeration |
- Chemical Functions
|
Property |
Effect of SOM |
|
Nutrient reservoir |
Source of N, P, S, and micronutrients |
|
CEC (Cation Exchange Capacity) |
Very high (150–300 meq/100g) |
|
pH buffering |
Resists sudden pH changes |
|
Chelation |
Forms complexes with Fe, Zn, Cu, Mn |
|
Detoxification |
Adsorbs heavy metals and pesticides |
- Biological Functions
|
Function |
Effect |
|
Microbial energy |
Provides carbon source for microorganisms |
|
Microbial habitat |
Creates favorable microenvironment |
|
Biological activity |
Enhances enzymatic and biochemical reactions |
|
Root growth |
Promotes better root environment |
- Environmental Functions
|
Function |
Effect |
|
Carbon sequestration |
Stores carbon, mitigating greenhouse gases |
|
Climate regulation |
Reduces CO₂ emissions |
|
Waste decomposition |
Assists in biodegradation of pollutants |
|
Water quality protection |
Reduces nutrient leaching and eutrophication |
- Distribution of SOM in Different Soils
|
Soil Type |
SOM (%) |
Remarks |
|
Peat soils |
20–90 |
Very high OM, poorly drained |
|
Forest soils |
3–6 |
Accumulated litter layer |
|
Grassland soils |
4–8 |
Deep organic-rich layer |
|
Alluvial soils |
0.5–2 |
Moderate SOM |
|
Arid soils |
<1 |
Very low SOM |
|
Black (Vertisols) soils |
1–3 |
High clay, good SOM retention |
- Maintenance and Improvement of SOM
Practices to Increase and Conserve SOM
- Add organic residues — FYM, compost, green manure, crop residues.
- Grow cover crops and legumes — Add root biomass and fix N.
- Adopt conservation tillage — Reduces oxidation of SOM.
- Avoid residue burning — Prevents carbon loss.
- Prevent soil erosion — Retains organic-rich topsoil.
- Balanced fertilization — Encourages biomass production.
- Integrated nutrient management (INM) — Combine organic and inorganic sources.
- Role of SOM in Soil Fertility
|
Aspect |
Role |
|
Nutrient source |
Provides 95% of soil N, 30–50% of soil P |
|
Soil reaction |
Buffers acidity and alkalinity |
|
CEC |
Retains cations, preventing nutrient leaching |
|
Structure |
Promotes aggregation and root growth |
|
Microbial life |
Provides food and habitat for soil organisms |
- SOM and Global Carbon Cycle
- SOM acts as both a source and sink of atmospheric carbon.
- CO₂ (atmosphere) ↔ Photosynthesis (plants) ↔ Plant residues ↔ SOM ↔ CO₂ (respiration)
- Soils store 2–3 times more carbon than the atmosphere.
- Enhancing SOM helps mitigate climate change by sequestering carbon.
