Soil Colloids (Inorganic and Organic)
- Introduction
- Soil colloids are the finest and most active particles in the soil.
- They are less than 1 µm (micrometer) in diameter (1 × 10⁻⁶ m).
- These particles have very high surface area and strong electrochemical activity, which make them responsible for nutrient retention, ion exchange, and soil structure.
- Definition; Soil colloids are very fine, amorphous or crystalline particles (both inorganic and organic) in soil that remain suspended in water and exhibit electrical charges on their surfaces, enabling them to adsorb cations and anions.
- Classification of Soil Colloids
Soil colloids are broadly classified into two main types:
|
Type |
Example |
Composition |
|
1. Inorganic Colloids (Mineral Colloids) |
Clay minerals, oxides of Fe and Al |
Silicates, oxides |
|
2. Organic Colloids |
Humus and its derivatives |
Carbon compounds (humic acids, fulvic acids) |
Inorganic (Mineral) Colloids
- Composition; Inorganic colloids mainly consist of clay minerals (silicate-type) and sesquioxides (Fe and Al oxides).
- Size: < 0.002 mm (or < 2 μm)
- Surface area: 10–800 m²/g
- Exhibit negative charge, hence attract cations (Ca²⁺, Mg²⁺, K⁺, etc.)
- Structure of Clay Minerals; Clay minerals are formed by the weathering of primary minerals like feldspars, micas, etc.
They are made up of two fundamental structural units:
|
Unit |
Composition |
Type of Sheet |
|
1. Tetrahedral Sheet |
Silicon (Si⁴⁺) surrounded by four oxygen atoms |
SiO₄ tetrahedra |
|
2. Octahedral Sheet |
Aluminum (Al³⁺) or Magnesium (Mg²⁺) surrounded by six oxygen/hydroxyl ions |
Al(OH)₆ or Mg(OH)₆ octahedra |
- Types of Clay Minerals (based on layer structure)
|
Type |
Layer Ratio |
Example |
Characteristics |
|
1:1 type |
1 T + 1 O |
Kaolinite |
Non-expanding, low CEC (3–15 meq/100g), stable structure |
|
2:1 type |
2 T + 1 O |
Montmorillonite, Smectite |
Expanding clay, high CEC (80–150 meq/100g), swelling & shrinkage |
|
2:1 type (non-expanding) |
2 T + 1 O |
Illite (mica-type) |
Moderate CEC (20–40 meq/100g), K⁺ fixed between layers |
|
2:1:1 type |
2 T + 1 O + 1 OH sheet |
Chlorite |
Non-expanding, moderate CEC (15–30 meq/100g) |
- Properties of Inorganic Colloids
|
Property |
Explanation |
|
Surface area |
Extremely high; controls reactivity and adsorption. |
|
Surface charge |
Mostly negative due to isomorphous substitution (e.g., Al³⁺ replacing Si⁴⁺). |
|
Cation Exchange Capacity (CEC) |
High ability to attract and hold cations on their surface. |
|
Swelling and Shrinkage |
Prominent in smectite-type clays (montmorillonite). |
|
Plasticity and Cohesion |
Due to attraction between water and clay particles. |
5. Organic Colloids
- Composition: Organic colloids are derived from decayed plant and animal residues, mainly in the form of humus.
- Size: < 1 μm
- Color: Dark brown to black
- Structure: Amorphous (non-crystalline)
- Charge: Predominantly negative (pH-dependent)
- Components of Humus
|
Component |
Characteristics |
Charge / Stability |
|
Humic acid |
Insoluble in acid, soluble in alkali |
Moderate negative charge |
|
Fulvic acid |
Soluble in both acid and alkali |
Highest CEC; smallest molecules |
|
Humin |
Insoluble in acid and alkali |
Least reactive, stable fraction |
- Properties of Organic Colloids
|
Property |
Description |
|
Surface charge |
Negative, pH-dependent (due to –COOH and –OH groups) |
|
CEC |
Very high (150–300 meq/100g) |
|
Water-holding capacity |
Extremely high due to colloidal nature |
|
Coloring effect |
Gives dark color to surface soils (A horizon) |
|
Stabilizing agent |
Helps in forming stable soil aggregates with clay and Ca²⁺ |
Comparison between Inorganic and Organic Soil Colloids
|
Characteristic |
Inorganic Colloids (Clay) |
Organic Colloids (Humus) |
|
Origin |
Weathered minerals (silicates, oxides) |
Decomposition of organic matter |
|
Structure |
Crystalline (layered) |
Amorphous (non-crystalline) |
|
Charge |
Permanent (isomorphous substitution) |
pH-dependent (functional groups) |
|
CEC |
3–150 meq/100g |
150–300 meq/100g |
|
Color |
Grey, white, red |
Dark brown to black |
|
Swelling |
Present in smectites |
Absent |
|
Solubility |
Insoluble in water |
Partly soluble (humic & fulvic acids) |
|
Stability |
Stable under most conditions |
Decomposes gradually |
|
Nutrient retention |
Moderate to high |
Very high |
|
Effect on structure |
Gives cohesion & plasticity |
Improves aggregation & porosity |
- Importance of Soil Colloids
- Nutrient Retention & Supply: Act as a reservoir of essential cations (Ca²⁺, Mg²⁺, K⁺, NH₄⁺). Exchange ions with soil solution as per plant needs.
- Soil Reaction (pH) Control: Colloids buffer soil pH by absorbing or releasing H⁺ and OH⁻ ions.
- Soil Structure Formation: Organic colloids help form stable aggregates by binding soil particles.
- Water-Holding Capacity: Colloids increase the field capacity and available water content of soil.
- Adsorption of Chemicals: Pesticides, metals, and organic molecules are held on colloidal surfaces, affecting mobility.
- Soil Fertility Indicator: High colloidal activity generally indicates fertile soils.
- Behavior of Soil Colloids in Relation to Soil Reaction (pH)
- At low pH (< 5.5) → H⁺ ions dominate → reduces negative charge (especially in humus & oxides).
- At high pH (> 7.0) → OH⁻ ions dominate → increases negative charge, enhancing CEC.
- Hence, pH-dependent charge is more significant in organic colloids and sesquioxides.
- Ion Exchange Phenomenon
- Cation Exchange: Replacement of one adsorbed cation by another on the colloid surface. Example: Clay – Ca+ 2Na+ ↔ Clay – Na2 + Ca2+
- Anion Exchange: Occurs in oxides of Fe and Al with positive surface charge under acidic conditions. Example: Fe – OH2++ Cl− ↔ Fe–Cl + H2O
Important Properties of Soil Colloids
The key properties of soil colloids that control their behavior and influence soil fertility are described below:
i) Surface Area
- Colloidal particles have enormous surface area per unit mass.
- Approximate surface areas:
|
Material |
Surface Area (m²/g) |
|
Sand |
0.01 |
|
Silt |
1 |
|
Clay (Kaolinite) |
10–20 |
|
Montmorillonite |
700–800 |
|
Humus |
800–1200 |
Importance: A greater surface area → more adsorption of water, nutrients, and cations → higher fertility and reactivity.
ii) Surface Charge
- Soil colloids carry electrical charges (mostly negative, sometimes positive).
- The negative charge arises mainly from:
- Isomorphous substitution in clay minerals (e.g., Al³⁺ replacing Si⁴⁺)
- Ionization of –COOH and –OH groups in organic matter
- The positive charge occurs due to: Protonation of hydroxyl groups in oxides of Fe, Al under low pH.
|
Colloid Type |
Charge Nature |
Cause of Charge |
|
2:1 clays (Smectite) |
Permanent negative |
Isomorphous substitution |
|
Fe, Al oxides |
Variable (positive/negative) |
Hydroxyl ionization |
|
Humus |
Variable negative |
–COOH and –OH dissociation |
iii) Adsorption
- Due to surface charge, colloids adsorb ions and polar molecules on their surface.
- The adsorbed ions are exchangeable (loosely held) and can move between the solid and liquid phases.
- This adsorption is reversible and forms the basis of ion exchange and nutrient availability.
iv) Ion Exchange Capacity; The ability of soil colloids to adsorb and exchange cations or anions with the soil solution is called ion exchange capacity.
Two main types:
- Cation Exchange Capacity (CEC) – exchange of positively charged ions
- Anion Exchange Capacity (AEC) – exchange of negatively charged ions
|
Soil Material |
CEC (meq/100g) |
|
Kaolinite |
3–15 |
|
Illite |
20–40 |
|
Montmorillonite |
80–150 |
|
Humus |
150–300 |
v) Plasticity and Cohesion
- Plasticity is the ability of wet clay to be molded and retain shape.
- Cohesion is the attraction among soil particles.
- Both depend on type of clay mineral and amount of water.
- Smectitic clays → high plasticity
- Kaolinitic clays → low plasticity
vi) Swelling and Shrinkage
- Some clays (especially montmorillonite) absorb large quantities of water between their layers, causing swelling.
- On drying, water escapes and the soil shrinks, forming cracks (typical of Vertisols).
Agricultural importance: Affects tillage, irrigation, root penetration, and soil aeration.
vii) Flocculation and Dispersion
|
Flocculation |
Dispersion |
|
Aggregation of colloidal particles |
Separation of particles |
|
Occurs when Ca²⁺ or Mg²⁺ dominate |
Occurs when Na⁺ dominates |
|
Promotes good soil structure |
Leads to poor structure (cloddy) |
Example: Gypsum (CaSO₄·2H₂O) is used to flocculate sodic soils by replacing Na⁺ with Ca²⁺.
viii) Buffering Capacity
- The ability of soil to resist sudden change in pH or nutrient concentration.
- Colloids adsorb or release H⁺, OH⁻, and nutrients to maintain equilibrium.
- High buffering soils = those with high clay and humus content.
ix) Colloidal Activity
- Colloids are the seat of soil chemical activity — where most nutrient retention, release, and microbial interactions occur.
- They act as reservoirs of nutrients available to plants.
Ion Exchange in Soils
- Ion exchange is one of the most important phenomena governing soil fertility and nutrient availability.
a) Definition: Ion exchange is the reversible process by which ions are exchanged between the solid phase (soil colloids) and the soil solution without any permanent change in the structure of the soil colloid.
b) Types of Ion Exchange
There are two major types of ion exchange in soils:
|
Type |
Description |
Example |
|
1. Cation Exchange |
Exchange of positively charged ions (cations) between soil colloids and soil solution |
Clay–Ca + 2Na⁺ ⇌ Clay–Na₂ + Ca²⁺ |
|
2. Anion Exchange |
Exchange of negatively charged ions (anions) between soil colloids (positive surfaces) and soil solution |
Fe–OH₂⁺ + Cl⁻ ⇌ Fe–Cl + H₂O |
c) Mechanism of Cation Exchange
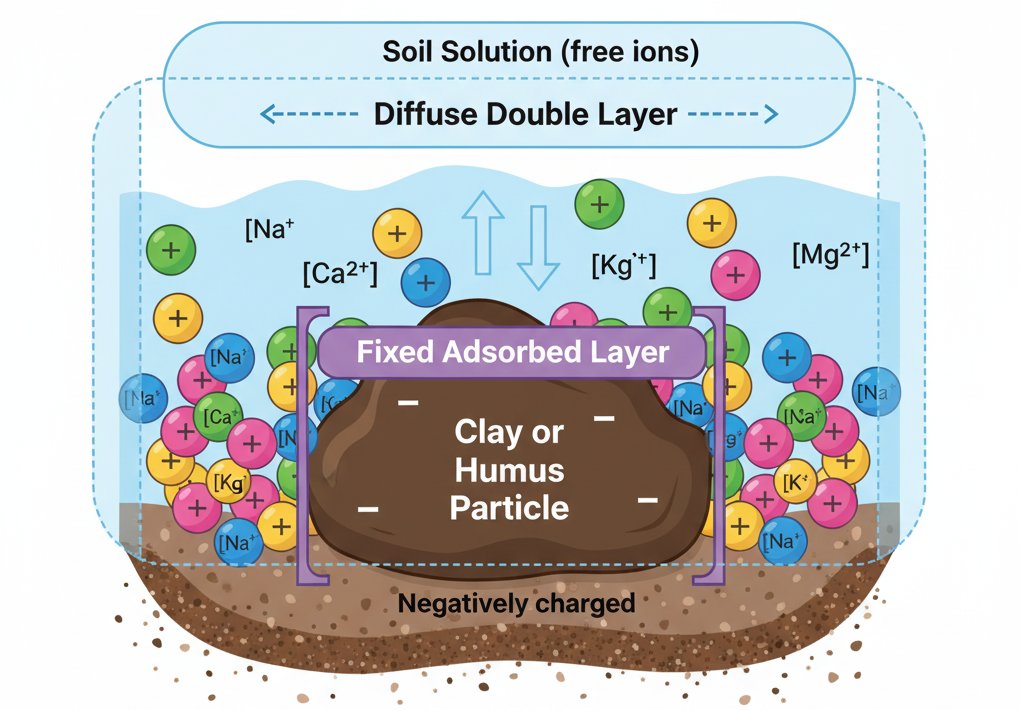
- Soil colloids (mainly clay and humus) carry negative charges.
- These attract and hold cations around them in a diffuse double layer (electrostatic field).
- The cations are not permanently fixed — they are in dynamic equilibrium with soil solution.
- Example reaction: Clay – Ca + 2Na+ ⇌ Clay–Na2+Ca2+
- This means: Ca²⁺ adsorbed on colloid can be replaced by Na⁺, and vice versa. The process is reversible and charge-balanced.
d) Reversibility of Ion Exchange
- Ion exchange reactions are rapid and reversible.
- The cation adsorbed on the exchange site can easily be replaced by another cation of equal charge if it is present in higher concentration.
e) Law of Mass Action: The ion exchange process follows the law of mass action, i.e., the rate and direction of exchange depend on the relative concentration of ions in the soil solution.
Higher the concentration of a particular ion in soil solution → greater its chance to replace a similar ion on the colloid surface.
e) Selectivity or Preference of Cations
- Soil colloids have a definite preference or selectivity for certain cations.
- Order of cation adsorption (lyotropic series):
- Al3+ > Ca2+ > Mg2+ >K+ = NH4+ >Na+
- Reason: Cations with higher valency and smaller hydrated radius are more strongly held.
f) Cation Exchange Capacity (CEC)
- The total capacity of a soil to hold exchangeable cations is called its Cation Exchange Capacity (CEC).
- Expressed in milliequivalents per 100 g of soil (meq/100g).
|
Soil Material |
CEC (meq/100g) |
Nature |
|
Kaolinite |
3–15 |
Low activity |
|
Illite |
20–40 |
Moderate |
|
Smectite |
80–150 |
High |
|
Humus |
150–300 |
Very high |
Agronomic importance: High CEC → better nutrient-holding and buffering capacity.
g) Anion Exchange
- Occurs in soils containing positively charged colloids, especially oxides of Fe, Al and hydroxides under acidic conditions.
- The mechanism involves hydroxylation and protonation of surface OH groups.
- Example: Fe – OH2+ + Cl− ⇌ Fe – Cl + H2O
- Importance: Retains important anions like phosphate (PO₄³⁻), sulfate (SO₄²⁻), and nitrate (NO₃⁻).
Agricultural Importance of Ion Exchange
- Nutrient Supply to Plants: Nutrients held on colloids are available through exchange with root uptake (Ca²⁺, K⁺, Mg²⁺, NH₄⁺).
- Fertilizer Efficiency: Fertilizer cations are retained and released gradually → prevents leaching losses.
- Soil Reaction Control: Liming and acidification depend on ion exchange of H⁺ and Ca²⁺.
- Soil Structure Maintenance: Ca²⁺ promotes flocculation; Na⁺ causes dispersion → affects tilth.
- Reclamation of Problem Soils: Sodic soils are treated with gypsum (Ca²⁺ replaces Na⁺ on exchange sites).
- Buffering Capacity: Maintains stable soil pH and nutrient equilibrium.
